- HOME
- History of Mitsubishi Tanabe Pharma
- The History of Mitsubishi Tanabe Pharma
The History of Mitsubishi Tanabe Pharma
The company's history began in 1678 when Tanabeya Gohei the 1st
opened a store in Tosabori, Osaka producing and selling Tanabeya
Infusing Medicine.
Going back further to 1604, Tanabeya
Matazaemon, the great-grandfather of Tanabeya Gohei the 1st, had
already started overseas trade, importing and introducing to Japan
many valuable medicinal materials from far off regions. For over
300 years since our founding, we have cherished and carried down
the spirit of our predecessors, crossing eras and national
borders, and have created many pharmaceuticals to contribute to
the healthier lives of people around the world.

|

Shogun Tokugawa leyasu issues to Tanabeya Matazaemon a Shuinjo (Shogunate license to trade) to travel to Luzon Kingdom (present-day the Philippines). |
|---|---|
 |

Tanabeya Gohei Ⅰstarts an independent business (later to become Tanabe Seiyaku) in Tosabori 1-chome, 0saka, manufacturing and selling medicinal compound "TANABEYA GUSURI (Tanabeya medicine)." |
 |

Tanabeya Gohei Ⅰis awarded the honorary title of "Kurokawa Yamato Daijo Fujiwara Kanenaga" for his services as purveyor to the Imperial Household. |
 |

Tanabeya Gohei Vl moves the business to Doshomachi 1-chome in Osaka, and becomes an official member of Yakushu Nakagai Kabu Nakama (Medicine Traders Guild). |
 |

Tanabeya Gohei Xl opens a new store in Doshomachi 3-chome (current location of Osaka Headquarter). |
 |

Tanabe Gohei Shoten obtains sole distribution rights for salicylic acid manufactured by German drug company Chemische Fabrik von Heyden, and begins to sell the product under the name "Hinode Tsurukame Jirushi sarichiru-san (Rising Sun, Crane, and Tortoise Mark salicylic acid)." |
 |
Tanabe Gohei Ⅻ opens Tanabe Motosaburo Shoten (later to become Tokyo Tanabe Seiyaku) in Nihonbashi-ku, Tokyo (present-day Nihonbashi area of Chuo-ku, Tokyo). |
 |

Tanabe Gohei Shoten constructs a factory in Honjo Kawasaki-cho, Kita-ku, 0saka to manufacture new pharmaceuticals, setting up a full-fledged domestic production system. |
 |

Tanabe Motosaburo Shoten launches its first new pharmaceutical, the fragrant castor oil Castarol, followed by topical analgesic/anti-inflammatory SALOMETHYL. |
 |

Tanabe Gohei Shoten establishes Onoda Seiyakusho(present-day Onoda plant)in Onoda, Yamaguchi Prefecture. |
 |
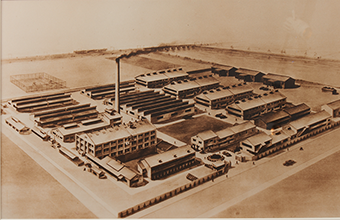
Tanabe Gohei Shoten constructs Osaka Plant as its main plant. |
 |
Takeda Chobei Shoten (later to become Takeda Pharmaceutical Company Limited) and Nippon Kasei form the 50/50 joint venture company Takeda Kasei Co., Ltd. (later to become Yoshitomi Pharmaceutical). |
 |

Takeda Kasei constructs its Yoshitomi Plant in the town of Yoshitomi in Fukuoka Prefecture. |
 |
Tanabe Gohei Shoten changes its name to Tanabe Seiyaku Co., Ltd. |
Tanabe Motosaburo Shoten changes its name to Tokyo Tanabe Seiyaku Co., Ltd. |
|
 |
Takeda Kasei changes its name to Yoshitomi Pharmaceutical lndustries, Ltd. |
 |

Tanabe Seiyaku launches anti-tuberculosis drug NIPPAS. |
Blood Plasma Corporation of Japan (later to become Green Cross Corporation) is established in Joto-ku, 0saka as the first blood bank in Japan. |
|
 |

Tanabe Seiyaku is awarded the 1st Deming Application Prize from the Union of Japanese Scientists and Engineers Deming Prize Committee. |
 |

Yoshitomi Pharmaceutical launches major tranquilizer CONTOMIN. |
 |

Tokyo Tanabe Seiyaku Co., Ltd. succeeds in the industrial production of ursodeoxycholic acid, and launches URSO to improve hepatic, biliary and digestive functions. |
 |
Tanabe Seiyaku signs a contract with the Research Foundation for Microbial Diseases of Osaka University (BIKEN) to market vaccines and antisyphilitic agents manufactured by BIKEN. |
 |

Tanabe Seiyaku launches topical corticosteroid FLUCORT Cream. |
 |

Tanabe Seiyaku launches aspartic acid drink ASPARA C. |
Blood Plasma Corporation of Japan changes its name to Green Cross Corporation. |
|
 |
Tanabe Seiyaku succeeds in the development and the world's first industrial applicalion of continuous production of L-amino acids using immobilized enzymes. |
 |
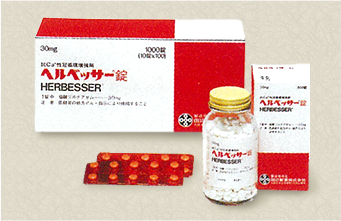
Tanabe Seiyaku launches continuous calcium antagonist HERBESSER. |
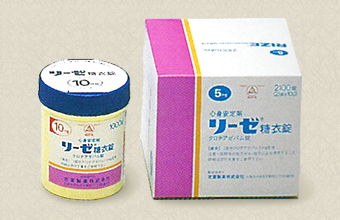
Yoshitomi Pharmaceutical launches antianxiety agant RIZE. |
|
 |
Yoshitomi Pharmaceutical launches antianxiety agant DEPAS. |
 |

Tokyo Tanabe Seiyaku launches topical synthetic corticosteroid cream MYSER as the frst product resulting from the partnership wlth Mitsubishi Kasei Corporation. |
 |
Tanabe Seiyaku receives the "Pharmaceutical Society of Japan Award for Drug Research and Development" for HERBESSER. |
 |
Tanabe Seiyaku launches selective β₁ antagonist MAINTATE. |
Tokyo Tanabe Seiyaku launches selective antithrombin agent NOVASTAN. |
|
 |

Tokyo Tanabe Seiyaku launches 5-HT₂ blocker ANPLAG. |
Tanabe Seiyaku launches selective angiotensin-converting enzyme inhibitor TANATRIL and gastritis/gastric ulcer remedy GASTROM. |
|
 |
Mitsubishi Kasei Corporation and Mitsubishi Petrochemical merge to establish Mitsubisi Chemical Corporation. |
 |
Yoshitomi Pharmaceutical Industries and Creen Cross Corporation merge, establishing Yoshitomi Pharmaceutical Industries. |
 |
Tokyo Tanabe Seiyaku launches hypercholesterolemic drug CHOLEBINE, which was originated by Mitsubisi Chemical Corporation and was the first product to be co-developed by the two companies. |
Mitsubishi Cemical and Tokyo Tanabe Seiyaku merge, and the pharmaceutical business is spun off into Mitsubishi-Tokyo Pharmaceuticals. |
|
 |
Yoshitomi Pharmaceutical Industries changes its name to Welfide Corporation. |
Tanabe Seiyaku launches selective histamine H1 receptor antagonist/anti-allergic agent TALION and oral dosing anti-spinocerebellar degeneration drug CEREDIST. |
|
 |
Welfide and Mitsubishi-Tokyo Pharmaceuticals merge, establishing Mitsubishi Pharma Corporation. |
 |
Tanabe Seiyaku launches anti-human TNFα monoclonal antibody preparation REMICADE. |
 |
Mitsubishi Pharma Corporation receives the "Pharmaceutical Society of Japan Award for Drug Research and Development" for RADICUT. |
 |
Mitsubishi Pharma Corporation and Mitsubishi Chemical Corporation jointly establish Mistubishi Chemical Holdings Corporation. |
 |
Tanabe Seiyaku and Mitsubishi Pharma merge to establish Mitsubishi Tanabe Pharma Corporation. |
 |
Mitsubishi Tanabe Pharma Corporation acquires the exclusive rights to market KREMEZIN, a chronic renal failure treatment. |
 |

Mitsubishi Tanabe Pharma Corporation launches OKINAZOLE L100, a treatment for recurrent vaginal candida. |
 |
Mitsubishi Tanabe Pharma Corporation launches SIMPONI Subcutaneous Injection 50mg Syringe, an agent for rheumatoid arthritis. |
Mitsubishi Tanabe Pharma Corporation launches IMUSERA Capsules 0.5 mg, a multiple sclerosis treatment. |
|
 |
Mitsubishi Tanabe Pharma Corporation receives the "Pharmaceutical Society of Japan Award for Drug Research and Development" for IMUSERA. |
Mitsubishi Tanabe Pharma Corporation launches TENELIA Tablets 20mg, a treatment for type 2 diabetes. |
|
Mitsubishi Tanabe Pharma Corporation launches TETRABIK subcutaneous injection syringe, a quatraple vaccine. |
|
 |
Mitsubishi Tanabe Pharma Corporation receives the "Pharmaceutical Society of Japan Award for Drug Research and Development" for CANAGLU. |
Mitsubishi Tanabe Pharma Corporation launches CANAGLU Tablets 100mg, a treatment for type 2 diabetes. |
|
 |

Completion of new head office building in Dosho-machi, Historical Museum included. |
CEREKINON S, a drug for improving recurrent symptoms of irritable bowel syndrome, is launched following the antecedently-launch in Kyushu and Okinawa in July 2014. |
|
 |

Tanabe Gastrointestinal Treatment URSO is launched. |
 |
Co-promotion of STELARA, a monoclonal antibody preparation, starts. |
Mitsubishi Tanabe Pharma Corporation launches CANALIA Combination Tablets, a treatment for type 2 diabetes. |
|
Mitsubishi Tanabe Pharma Corporation launches RUPAFIN Tablets 10mg, an allergic disease treatment. |
|
 |
Mitsubishi Tanabe Pharma Corporation launches VAFSEO Tablets 150mg and VAFSEO Tablets 300mg, treatments for renal anemia. |
TALION AR, a drug exclusively for allergic rhinitis, is launched. |
|
 |
Mitsubishi Tanabe Pharma Corporation launches UPLIZNA for Intravenous Infusion 100mg, a treatment for neuromyelitis optica spectrum disorders. |
 |
Mitsubishi Tanabe Pharma Corporation launches DYSVAL Capsules 40mg, a treatment for tardive dyskinesia. |
RADICAVA ORS, an oral suspension for the treatment of ALS, is launched in the U.S.. |
|
 |
Mitsubishi Tanabe Pharma Corporation launches Mounjaro® Ateos®, GIP/GLP-1 receptor agonist. |
 |
Mitsubishi Tanabe Pharma Corporation launches GOBIK Aqueous Suspension Syringes, the combination vaccine for five diseases. |
Mitsubishi Tanabe Pharma Corporation launches Alesion Eyelid Cream 0.5%, a new treatment for allergic conjunctivitis. |